Science-based / data-driven material production
We position material production within the PDCA cycle of pharmaceutical marketing,
enhancing content value through a science-based / data-driven perspective.
Quality-focused material production by a “small but elite” team
Our team consists of staff with extensive industry experience, ensuring a high level of quality standardization.
We fully understand product marketing and academics, and will reliably execute material production.
-

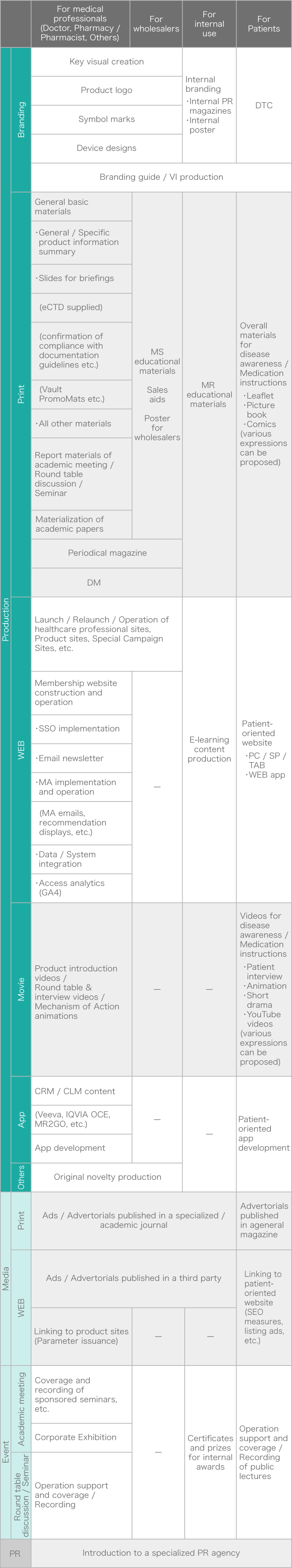
General material production
We ensure reliable material production including review responses in compliance with documentation guidelines and company regulations.
Considering the product strategy, we propose the optimal materials - whether print or digital -, based on your budget and deadline.
At launch, we can handle all kinds of materials, from the basic materials based on CTD to academic materials, visual materials, etc. We can also handle all related tasks, such as obtaining permission to reproduce, requesting supervision, and making honorarium payments. -

Video and animation production
We can produce various types of content, including mechanism-of-action animation, clinical results presentation videos, doctor interview videos, and YouTube videos. Based on your product strategy, we can handle everything from draft / storyboard to talent auditions / casting, location shooting, CG production, animation production, video editing, MA, and final delivery.
-

Membership site development and operation, MA implementation, data / system integration
Entrust us with everything from owned site launch, modification, and feature expansion to ongoing monthly operations.
We have extensive experience in complex development projects, including backend production and data / system integration with existing systems. -

Product branding
We develop visual expressions that maximize product value during product launches and rebranding phases.
We analyze from every angle - product characteristics, disease traits, competitive differentiation - to create key visuals, symbol marks, and device designs. -

CRM / CLM
We maximize the capabilities of CRM / CLM systems, including Veeva, to create content and design / build data integration specifications.
Collaboration with Veeva official partners enables smooth and secure development. -

Data analysis and utilization
We objectively identify issues across the entire site and individual content by analyzing owned site traffic using GA4 and MA tools, email newsletter open rates / traffic sources, and user content evaluations. This enables us to continuously improve material production through the PDCA cycle.
Solving client challenges
We provide practical solutions to the concerns and marketing challenges
that pharmaceutical companies face with their existing agencies.
-
Concerns regarding existing agencies

Agency selection
You look for an agency capable of handling all kind of material production and managing owned site operations and content end-to-end in collaboration with SIers.
You selected main agency based on an attractive proposal in an agency competition, but the actual staff handling the work are underqualified and struggle even with basic material production.
Contractually, you cannot change your main agency, but you are seeking a secondary agency that can compensate for the main agency's weaknesses.The existing agency's performance is poor, and you are considering a review.
Production coordination & proposals
The existing agency's responses are slow. Even when they finally reply, it’s often irrelevant.
The front-line account planner's level is low. It’s straight to the point to talk with the director directly.
There are no major issues with routine standard tasks, but the existing agency’s lack of ability to propose solutions to leaves you feeling unsatisfied when you need solutions to your challenges.
Review responses
The agency is unable to devise countermeasures for review comments, placing a heavy burden on the persons in charge of material production.
Due to the agency's sloppy work, the persons in charge of material production must handle everything - attaching red-lined revisions, communicating the intent behind corrections - leaving them exhausted.
-
Marketing challenges

Owned site
Several years have passed since introducing a membership system to the owned site, but membership growth has stagnated.
In addition, the member activity rate has been declining over time.While you are attempting to acquire new members through third-party advertising and webinars, there is room for improvement in member acquisition through site navigation.
(e.g., low pages per session)Conversion settings for the owned site operations are unclear.
Email newsletters
Email newsletters open and click rates are stagnating.
You regularly send out email newsletters, but they are limited to specific segments, and you are unable to tailor them to meet customer attributes and behavior.
MA, CRM
You implemented a MA tool, but you haven't been able to utilize it effectively.
In addition, proposals from SIers are too technically focused, placing a heavy burden on the person in charge of implementing them as measures.Hot lead information in the digital realm is not being promptly integrated into MR activities.
Approved email
You have not yet considered what content should be attached and sent other than the webinar.